How Much Chlorine To Inject to Treat Iron & Manganese Before Iron Filters?
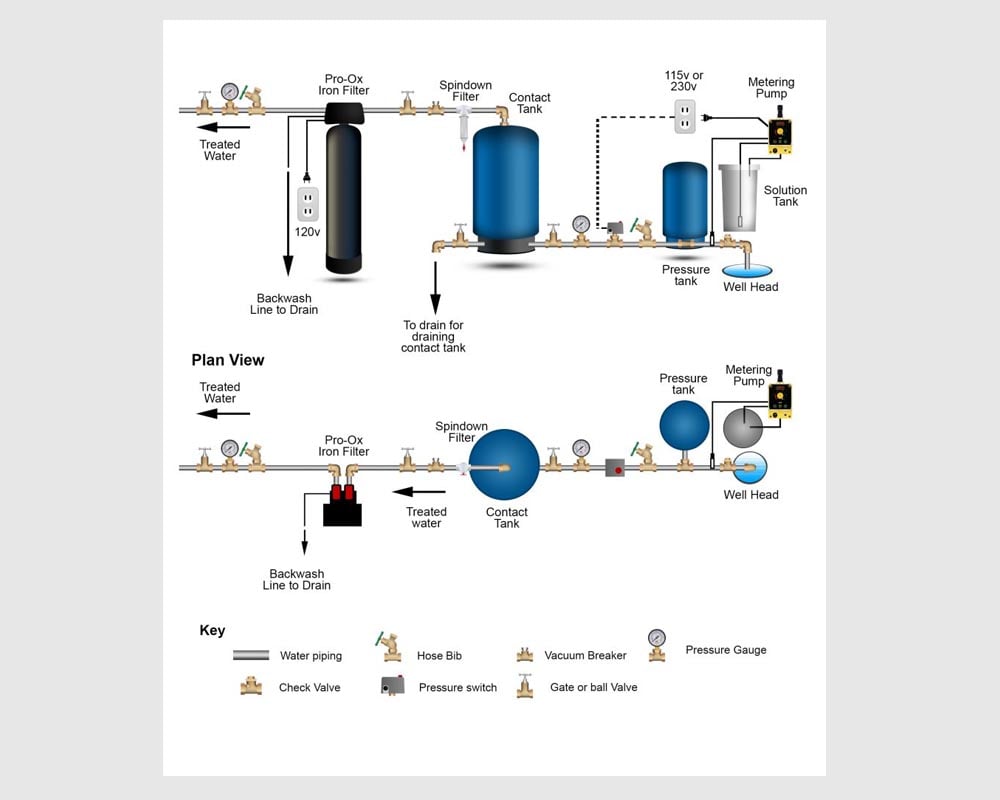
Chlorine Injection for Iron Removal: How Much To Inject Before Iron Filters?


A common question we often get is, how much chlorine do I need to inject before my iron filter to treat iron, manganese, and hydrogen sulfide (rotten egg odor)?
The short answer is:
- 0.62 mg/l of chlorine per mg/l of iron;
- 1.29 mg/l of chlorine per mg/l of manganese
- 8.33 mg/l of chlorine per mg/l of sulfide
Note that mg/L (milligram per liter) is the same as saying PPM (parts per million).
Pre-Treatment With Chlorine and Filtration with Pro-OX Manganese Dioxide Media
A rapid and efficient method for removing iron, manganese, and hydrogen sulfide is chlorinating the raw water, allowing a 30–60 second retention time, and filtering with Pro-OX manganese dioxide filter media. This process is a common form of chlorine injection for iron removal. A retention tank often removes the oxidized contaminants after chlorine treatment.
To inhibit the growth of iron and sulfur bacteria, a level greater than 0.5 mg/l of oxidant (chlorine) should be injected into the storage tanks and distribution system.
Water is received from the well source and treated with chlorine to oxidize the iron, manganese, and sulfides present. The iron is oxidized to ferric iron, the manganese to the manganic form, and the sulfides to sulfate.
Sulfate is common in water supplies and does not contribute to an objectionable taste or odor. A sufficient amount of chlorine is added to the water to meet the chemical demand and reach the chlorine breakpoint. The distribution system receives a minimum free chlorine residual of 0.5 mg/l. Depending on conditions, a higher residual may be injected.
How Much Chlorine To Inject?
Stoichiometry is used to determine the number of reactants (in this case, chlorine) needed in a given reaction. It describes the quantitative relationships among substances as they participate in chemical reactions.
The stoichiometric equivalent for chlorine needed to oxidize iron, manganese, and sulfide is:
- 0.62 mg/l of chlorine per mg/l of iron;
- 1.29 mg/l of chlorine per mg/l of manganese
- 8.33 mg/l of chlorine per mg/l of sulfide
- Plus, organic demands may be present, determined by jar testing or field pilot tests.
Plus, organic demands may be present, determined by jar testing or field pilot tests.
If your water contains both iron and manganese, chlorine injection for iron removal is generally more effective than hydrogen peroxide, which does not work well with manganese present.
For a more technical look at chlorine oxidation in iron and manganese removal, see this research article on ScienceDirect, which explores how chlorine interacts with various contaminants during water treatment.
Understanding Iron and Manganese in Drinking Water
Iron and manganese are naturally occurring elements found in drinking water. While they are essential for human health in small amounts, excessive levels can cause problems. Iron can give water an unpleasant taste, odor, and color, while manganese can cause discoloration and staining. Both elements can also contribute to the growth of iron bacteria, which can lead to further issues.
Iron bacteria are small living organisms that combine iron or manganese with oxygen to form deposits of “rust,” bacterial cells, and slimy materials. These deposits can damage well casings, pumps, pipes, plumbing fixtures, irrigation systems, and water appliances. Iron bacteria can also produce unpleasant tastes and odors, such as “swampy,” “oily,” “cucumber,” “sewage,” “rotten vegetation,” or “musty.”
Manganese, on the other hand, can cause neurological problems and other health issues if consumed in large amounts. It is essential to test your drinking water for iron and manganese to determine if they are present in excessive levels.
Importance of a Water Report
A water report is a detailed chemical composition analysis of your drinking water. It provides information on the levels of various contaminants, including iron, manganese, and bacteria. A water report is essential for determining the best course of treatment for your drinking water.
A water report can help identify potential health risks associated with your drinking water. For example, high levels of iron or manganese can indicate the presence of iron bacteria, which can lead to further issues. A water report can also help you determine the most effective treatment method for your drinking water.
Chlorine Injection for Iron Removal
Chlorine injection is a common method for removing iron from drinking water. Chlorine is added to the water to oxidize the iron, making it easier to remove. The oxidized iron is then filtered out of the water using an iron filter.
Chlorine injection is an effective method for removing iron from drinking water. However, the correct dosage of chlorine must be used to avoid excess chlorine, which can cause unpleasant tastes, odors, and health problems.
Injection System Considerations
Several factors must be considered when considering an injection system for iron removal. First, it is essential to determine the correct chlorine dosage. This will depend on the level of iron in your drinking water and the flow rate of your water system.
It is also important to consider the type of injection system to use. Several types of injection systems are available, including manual and automatic systems. Automatic systems are generally more convenient, as they can be programmed to inject the correct dosage of chlorine at set intervals.
Finally, it is essential to consider the injection system's maintenance requirements. Regular maintenance is necessary to ensure that the system is functioning correctly and that the water is safe to drink.
Recent Posts
Water Quality for Horses and Livestock: A Guide to Healthier Barns and Pastures
Clean water is the cornerstone of animal health and productivity. Whether you're raising horses, cattle,…
Clean Drinking Water for Cats: What Every Cat Owner Should Know
Why Clean Drinking Water Matters for Cats Hydration is critical to a cat’s overall health,…
Pet Hydration: What to Know About Water Safety
Clean Drinking Water for Dogs: What Every Dog Owner Should Know Clean drinking water is…
How to Ensure Fresh, Safe Water for Your Birds: Daily Care Tips for Bird Owners
Why Filtered Water for Birds Is Essential for Their Health Like food, clean drinking water…
U.S. Water Problems by Region: Common Contaminants & Solutions
Curious about U.S. water problems by region? Water quality isn’t just a national issue—it’s a…
Wildfire Water Contamination: How to Ensure Safe Water After a Fire
Wildfire Water Contamination: What You Need to Know After the Fires Drinking water contamination is…


