Can Hydrogen Peroxide Be Used to Remove Manganese and Iron from well water?
Can Hydrogen Peroxide Be Used to Remove Manganese and Iron from well water?
Recent studies have examined the effectiveness of different methods of removing manganese from well water. A graduate student at the University of Pittsburgh conducted a thorough study on this topic. It is important to store hydrogen peroxide in a well-ventilated area to minimize risks, as its stability and behavior in an aqueous solution can vary.
The project worked to figure out the optimal treatment method, which resulted in the removal of the most manganese and the smallest amount of disinfectant byproduct. An appropriate injectant must be used to reduce the number of electrons each ion has to break down ions such as manganese and iron. Occupational safety guidelines emphasize the importance of handling hydrogen peroxide with care to prevent exposure risks, including the potential for spontaneous combustion when it encounters organic materials.
The substance that receives the extra electrons is called the oxidizing agent. Hydrogen peroxide is used in various industrial processes, including bleaching, cleaning, and disinfecting.
The study found that “Hydrogen Peroxide at these [varying] pH levels was not an adequate oxidizing agent for manganese. Higher concentrations of hydrogen peroxide can be more hazardous. Iron, however, was oxidized when hydrogen peroxide was present. Interactions with other chemicals in industrial applications can also influence its effectiveness and safety.
In conclusion, using hydrogen peroxide was not beneficial. Peroxide did not oxidize the manganese, and it created massive complications with chlorine used for disinfection. Hydrogen peroxide interacts with organic material during its application, particularly affecting tissues containing organic elements like pus and blood.
The chart above shows the manganese removal results from different injections. One interesting point about the chart is that it takes over 100 minutes for chlorine to perfectly oxidize manganese. If not used properly, hydrogen peroxide can cause serious side effects.
But if chlorine is combined with our Pro-OX manganese dioxide media iron filters, or Greensand iron filters, the manganese is effectively removed by a catalytic reaction between the filter media and the chlorine, which results in manganese getting instantly oxidized on the surface of the filter media. Hydrogen peroxide is an active ingredient in various products. A strong backwash (10 GPM is OK) is needed to flush out the accumulated manganese oxides.

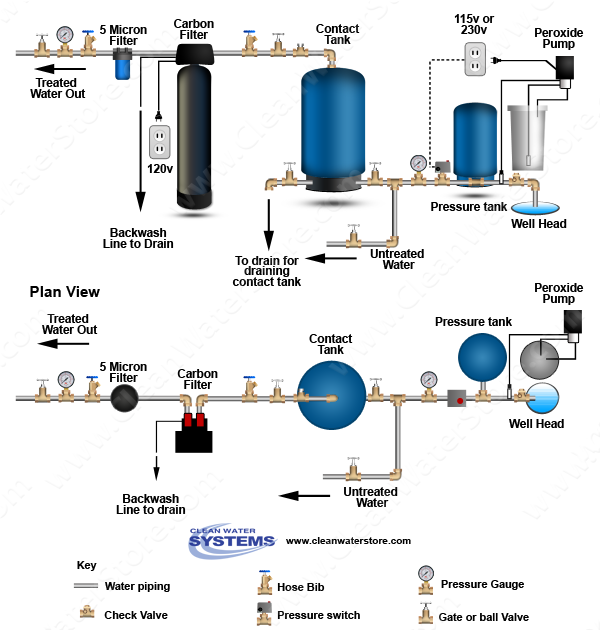
Below is a diagram showing how the system is set up. Official publications on hydrogen peroxide safety are available through the government printing office.
Follow this link to see the full thesis. The molecular weight of hydrogen peroxide is an important chemical property.
Below is a diagram showing the way the system is set up.
What is Hydrogen Peroxide?
Definition and Composition of the Colorless Liquid
Hydrogen peroxide is a chemical compound with the formula H2O2. Anhydrous hydrogen peroxide can be obtained through processes like vacuum distillation and is significant in various chemical reactions and treatments. It is a colorless liquid that is slightly more viscous than water. Known for its powerful oxidizing properties, hydrogen peroxide is widely used as a disinfectant, bleaching agent, and antiseptic. Various methods, including the AO/Antraquinone method, involve hydrogen and oxygen reacting to form hydrogen peroxide. The compound consists of two hydrogen atoms and two oxygen atoms, forming a molecular structure characterized by a nonplanar, twisted C2 symmetry. This unique structure contributes to its effectiveness in various chemical reactions, including its bleaching effect on organic materials, making it a versatile and valuable chemical in both household and industrial applications. Understanding these processes and properties is crucial in the field of chemical technology, which provides insights into hydrogen peroxide's industrial applications.
Uses of Hydrogen Peroxide
Household Uses
-
Wound care: Due to its antiseptic properties, a 3% hydrogen peroxide solution is commonly used to clean minor cuts and scrapes.
-
Mouth rinse: Helps remove mucus and relieve minor mouth irritation.
-
Surface disinfectant: Effective for sterilizing countertops and bathroom surfaces.
-
Hair and clothes bleach: Used for its bleaching capabilities in both personal care and laundry.
-
Agricultural uses: Occasionally applied in small-scale farming for disinfecting tools or treating fungal growth.
-
Environmental presence: Found naturally in the air and in small amounts in various household products.
-
Safety note: May cause skin irritation; proper handling and storage are essential to maintain effectiveness.
Industrial Uses
-
Rocket fuel component: Used in higher concentrations for propulsion systems.
-
Textile, pulp, and paper industries: Acts as a bleaching agent for fabrics and paper products.
-
Environmental protection: Used as a substitute for chlorine in water and sewage treatment.
-
Disinfection: Ensures hygiene in industrial and commercial settings.
-
Chemical manufacturing:
-
Produces organic compounds like dibenzoyl peroxide.
-
Involved in making mild bleaches such as sodium percarbonate and sodium perborate for detergents.
-
Used in producing inorganic peroxides essential for various applications.
-
-
Foam rubber production: Plays a role in manufacturing processes for flexible materials.
Properties of Hydrogen Peroxide
Physical Properties
Hydrogen peroxide is a colorless liquid with a characteristic pungent odor, making it easily identifiable. This compound has a low molecular weight of 34.014 g/mol, which contributes to its unique properties. One of the notable physical properties of hydrogen peroxide is its boiling point, which has been extrapolated to be 150.2 °C (302.4 °F). As a powerful oxidizing agent, hydrogen peroxide is widely used as a bleaching agent, disinfectant, and oxidizing agent in various applications. Its ability to decompose readily into water and oxygen makes it a versatile and effective compound in both household and industrial settings.
Chemical Properties
Chemically, hydrogen peroxide is a weak acid that can form hydroperoxide or peroxide salts with many metals. This ability to convert metal oxides into corresponding peroxides is a key aspect of its chemical behavior. The redox properties of hydrogen peroxide are highly dependent on the pH of the solution. In acidic conditions, H2O2 acts as a powerful oxidizer, making it effective in various oxidation reactions. Conversely, under alkaline conditions, it functions as a reductant. This dual capability as a strong oxidizing agent allows hydrogen peroxide to oxidize a wide range of organic and inorganic compounds, highlighting its versatility in chemical processes.
Risks and Precautions
Risk of Exposure
Hydrogen peroxide is classified as a toxic substance, and exposure to it can lead to severe health issues. Inhaling hydrogen peroxide solutions, especially those with concentrations over 10%, can cause severe pulmonary irritation. This risk underscores the importance of using hydrogen peroxide in a well-ventilated area to minimize inhalation hazards. Swallowing hydrogen peroxide solutions is particularly dangerous, as the decomposition of the compound in the stomach releases large quantities of gas, which can be harmful. High-concentration hydrogen peroxide streams, typically above 40%, are considered hazardous due to their classification as DOT oxidizers. The Environmental Protection Agency (EPA) has set the Reportable Quantity (RQ) for D001 hazardous wastes at 100 pounds (45 kg), or approximately 10 US gallons (38 L), of concentrated hydrogen peroxide. These guidelines emphasize the need for careful handling and storage to prevent accidental exposure and ensure safety.